The development of the product development process
There has been a significant change in the development of new products in the healthcare industry over the last few years. The influence of IT, new tools, technologies, and the genome has enriched the development possibilities and irreversibly changed the clinical trials environment. Embracing a more patient-centric mindset and embedding innovation is central to this transformation. This requires collaboration from all stakeholders and an exchange of information in real time.

Successful psoriasis treatment based on natural extracts
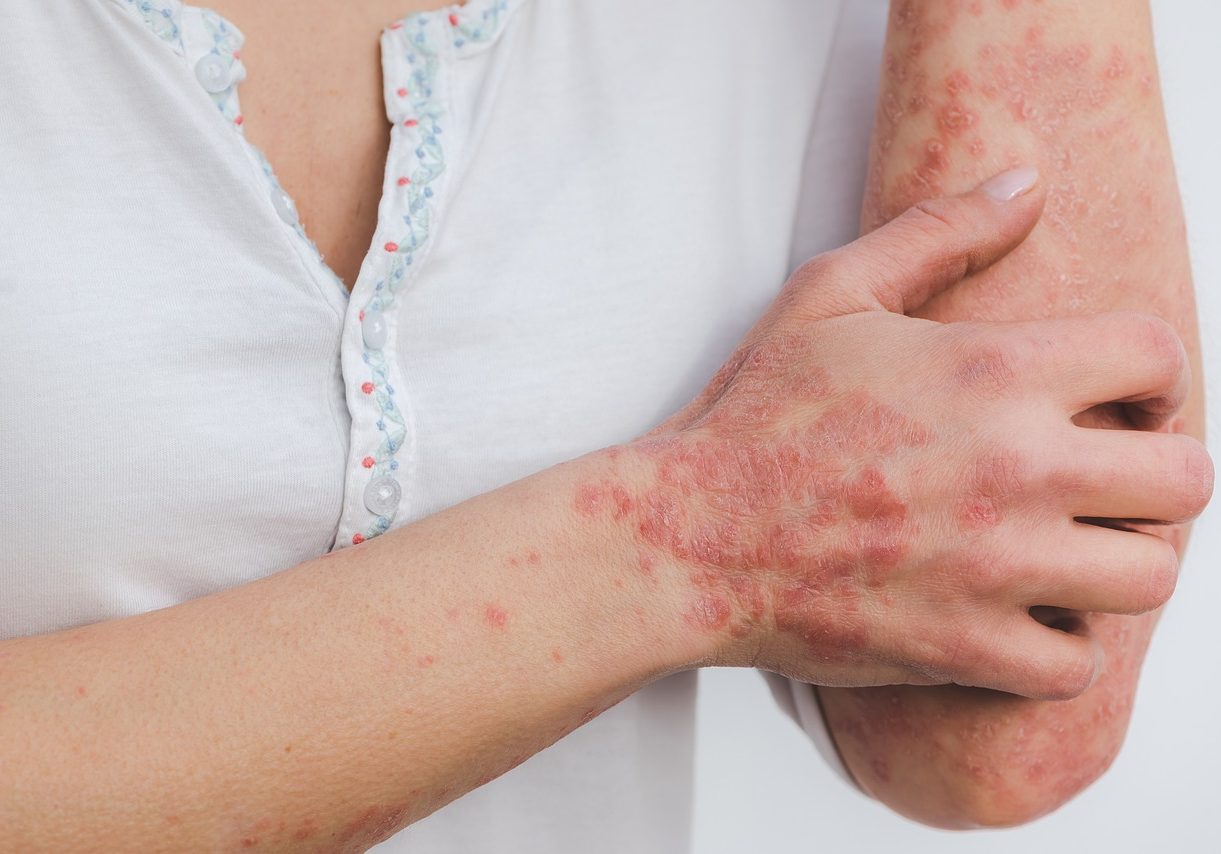
The current treatment of psoriasis is not satisfactory and includes a vast number of different therapeutic approaches ranging from topical and systemic agents to medical devices and gadgets. A new topical remedy based on natural extracts, and with an excellent safety profile is in development.
New Diagnostic Test to evaluate the risk of disorders during pregnancy

A new development of a home test to be used during pregnancy to identify potential risks. This test could potentially save lives of both women and babies.
Intelligent Antibiotic
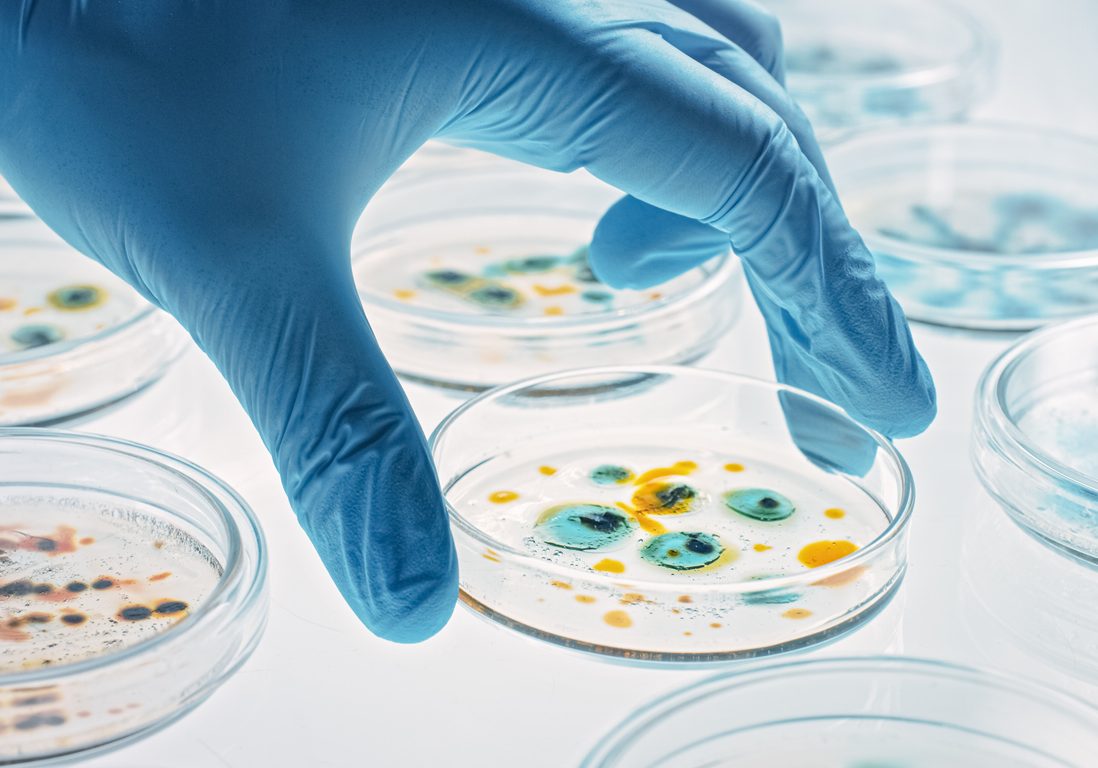
One of the biggest problems in today’s healthcare is the treatment of infectious diseases, not only in viral epidemics such as COVID-19, but also in the development of antibiotic bacterial resistance, and with this the need to develop new antibiotic therapies to counteract this challenge.
Immune Precision Medicine for Cancer
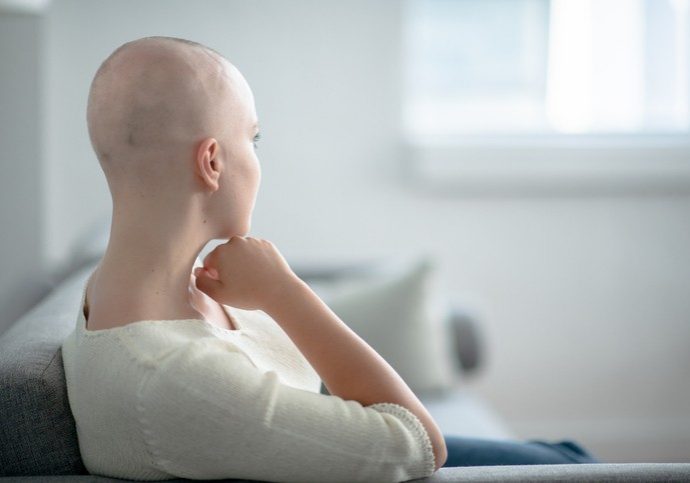
Cancer cell growth is associated with immune surveillance failure. Nowadays, restoring the desired immune response against cancer cells remains a major therapeutic strategy. IDRE is involved in the search for a new product.
Crohn’s Disease and Ulcerative Colitis

Intestinal inflammatory diseases are conditioned by multiple factors and depend on a balance between biota and intestinal epithelial membrane at different levels, as well as psychological and other external factors. Developing products that can tackle this balance is a promising therapeutic approach.
New less-addictive Pain Control

Pain is the most common alarm signal in our body. Even though the treatment of acute pain is well under control, in diseases with chronic pain several complications can obscure the results and at times make it intolerable. Addressing this aspect, preventing addiction and improving results using novel pain control therapies is the focus of our long-term projects.
The role of platelet factors in Cancer

Platelets have for many years been considered static elements without a nucleus and were therefore believed to have little activity. Recently, however, researchers have discovered that platelets are related to cancer and cell division. This fact is yet to be considered in oncology and is the source of a new therapy strategy.